The University of Oxford plans to test its AstraZeneca-produced COVID-19 vaccine on children for the first time, it was announced on Saturday.
The trial seeks to recruit 300 volunteers between the ages of 6 and 17, with up to 240 receiving the COVID-19 vaccine and the remainder a control meningitis vaccine.
The trial will begin this month at the university and additional sites in London, Southampton and Bristol, Sky News reported.
Andrew Pollard, chief researcher on the Oxford vaccine trial, says that while most children don’t get severely ill from COVID-19, “it is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit from vaccination.’’
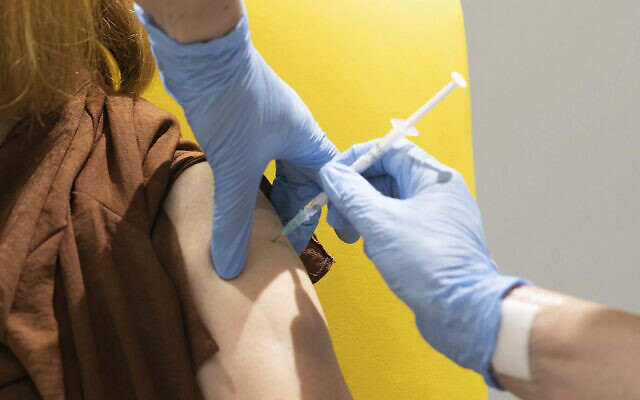
Undated file photo shows a volunteer receiving the coronavirus vaccine developed by AstraZeneca and Oxford University, in Oxford, England. (John Cairns/University of Oxford via AP, File)
Regulators in more than 50 countries have authorized widespread use of the Oxford vaccine, which is being produced and distributed by AstraZeneca, for use in people over the age of 18.
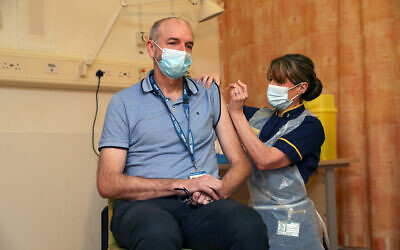
Professor Andrew Pollard, Director of the Oxford Vaccine Group, and a professor of paediatric infection and immunity receives the Oxford University/AstraZeneca COVID-19 vaccine at the Churchill Hospital in Oxford, Jan. 4, 2021 (Steve Parsons/Pool Photo via AP)
Other drug companies are also testing the COVID-19 vaccines in children. Pfizer, whose vaccine has already been authorized for use in people 16 and older, began testing its shot in children as young as 12 in October. Moderna in December began testing its vaccine on children as young as 12.
Pollard said the Oxford trial should help policymakers decide whether at some point in the future they want to extend mass vaccination programs to children as they seek to ensure schools are safe and combat the spread of the virus in the wider population.
“For most children, for themselves, COVID is really not a big problem…,’’ Pollard told The Associated Press. “However, it is certainly possible that wider use to try and curb the progress of the pandemic might be considered in the future, so here we’re just trying to establish the data that would support that if indeed policymakers wanted to go in that direction.”
Although children are generally at less risk of severe disease after contracting the coronavirus, a pediatrician and clinician-scientist at the Oxford Vaccine Group said that it was important to test the vaccine on children due to the negative effect of the pandemic on their education and emotional wellbeing.
“The COVID-19 pandemic has had a profound negative impact on the education, social development and emotional wellbeing of children and adolescents, beyond illness and rare severe disease presentations,” Rinn Song told the Guardian. “It is therefore important to collect data on the safety and the immune response to our coronavirus vaccine in these age groups, so that they could potentially benefit from inclusion in vaccination programs in the near future.”
 RSS Feed
RSS Feed















 February 13th, 2021
February 13th, 2021  Awake Goy
Awake Goy  Posted in
Posted in  Tags:
Tags: 













