Someone dies of a stroke every 4 minutes in the United States. Globally, 15 million people suffer a stroke every year. It’s debilitating physically and financially—but one little-known company has developed a medical device that hopes to challenge that deadly statistic.
It’s been quietly developing this new technology for 10 years. Now it’s released updated preliminary clinical trial results, and signed a manufacturing deal which could make a significant impact in the medical device industry.
After a decade of painstaking development, we’re now nearing the end of the long road to validation, and for investors who understand this industry, this could be the critical juncture.
The little-known company is CVR Medical (TSX:CVM.V; OTC:CRRVF), and its potentially life-saving device is the Carotid Stenotic Scan (CSS)–a technology designed to detect stenosis within arteries, or Ischemia, which is the leading indicator of strokes.
Of the 15 million people who suffer stroke every year, some 6 million are killed, while 5 million are rendered permanently disabled, according to the World Heart Foundation.
But there has been no cost-effective way to screen for Ischemia.
So, when a biotech company offers a potential solution to help prevent the second-leading cause of death in the world–and then releases positive preliminary clinical trial results, investors listen.
Now it’s hoping to charge out of the gate and take the market by storm once it manages to gain FDA market clearance.
In the meantime, the catalysts are really lining up:
On 7 September, CVR released updated results from its preliminary clinical trial that showed forward progress for the medical device, which we’ve been watching closely for some time. You can view the results from Thomas Jefferson University here.
And a few weeks prior, they announced another landmark achievement when they signed a letter of intent to manufacture the CSS. The deal gives CVR state-of-the-art manufacturing capabilities with Canon Virginia, Inc (CVI). This would give CVR immediate scalability, and also adds to its credibility: It’s not the first manufacturing deal the company has sealed.
Our researchers are keeping a close eye on CVR Medical Corp. because we think this is the turning point. Here’s why:
• It’s had two fresh catalysts in three weeks in an industry that is all about validation
• It’s developing a device that meets critical demand in terms of helping to prevent one of the most debilitating and deadly diseases of our time
• It’s developing a medical device that actually makes sense to the market
• It’s been doing it quietly, without blockbuster hype, and instead painstakingly going for real validation, making it the real deal.
Here are 5 reasons to keep a close eye on CVR Medical (TSX:CVM.V; OTC:CRRVF) in the coming weeks and months:
#1 Catalysts are Lining Up, and News Flow is Gaining Momentum
First, one way to look at the CSS is to think about what 3D seismic imagery did for super quick discoveries in the oil and gas industry. This is exactly what CVR’s sensory system could do for the medical industry in terms of detecting critical stroke symptoms.
This is how it works:
CVR’s Carotid Stenotic Scan (CSS) is a tool to detect blockage within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner.
The CSS makes a connection between fluid flow and low frequency soundwaves to detect arterial disease or blockage. Blood flowing through the carotid arteries produces wave patterns which are shaped and altered by the presence of irregularities on the inner artery walls.
CVR’s advanced technology captures these wave patterns and analyzes them mathematically with patented algorithms. After a brief test, the analysis is complete, offering a way to potentially identify those at risk of a stroke and arming the healthcare provider with the information necessary to prevent the deadly event.
Unlike other comparative modalities, the CSS was designed to function without the assistance of a certified technician. These three facts combine to create one of the potentially biggest—and most lucrative–phenomena in recent medical equipment market history.
The updated results of the preliminary clinical trials released just now bring the benefits of this device that much closer. Not only could it potentially help prevent strokes through early detection of arterial disease, but there is a secondary benefit: if all goes according to plan, it makes testing cheap, affordable and accessible to all.
#2 CSS Makes Market Sense at $49,000 Per Unit
Detecting the early signs of a stroke—before it’s too late—is a major challenge for our medical establishments. Often diagnoses are wrong. Usually, they are expensive.
CVR Medical’s (TSX:CVM.V; OTC:CRRVF) device, in late phase clinical trials, is expected to cost an estimated $49,000 per unit for detecting Ischemia, again—the leading indicator of stroke.
CT scans are the most common method of diagnosing a stroke—but usually after it’s already happened, and even then, stroke is not always seen on CT scans. CT scans can predict risk of stroke in patients who have suffered transient ischemic attacks (TIA), or ‘mini-strokes’, but its costs anywhere from $825 to $4800 for a CT scan, depending on your doctor and your insurance. That’s because CT scanners cost between $1 million and $2.5 million dollars each.
Coming in at the planned $49,000 per unit, compared to up to $2.5 million, CVR Medical’s CSS makes market sense. But beyond that, the CT scan isn’t enough for detection.
According to the Centers for Disease Control (CDC), early action is vital for survival, and only 38 percent of stroke sufferers even recognize they are having a stroke in time to receive effective emergency intervention.
#3 Urgent Need, Hungry Market
Few things are more urgent than an early detection system for a medical condition that kills 6 million people a year. In the U.S. alone, one American dies from stroke every 4 minutes, and right now the U.S. death toll from strokes stands at upwards of 130,000 each year. Annually, more than 795,000 Americans suffer a stroke.
“Strokes will absolutely strain the healthcare system,” says Bruce Ovbiagele, M.D., M.Sc., professor and chairman of the Department of Neurology at the Medical University of South Carolina, Charleston. Caring for survivors is expensive because stroke can cause long-term disability, he said. “Policy makers at all levels of governance should be aware of this looming crisis so that we can consider practical ways to avert it.”
Against the backdrop of these devastating statistics, CVR is hoping to make a dramatic impact upon an industry starved for innovation and advancement.
The CSS is a groundbreaking tool that can assess Carotid Arterial health in a way that has never been available to a patient, healthcare provider, or the payor in our current healthcare system.
There are 5,564 hospitals in the U.S. alone, in addition to the over 200,000 primary care physicians—many operating primary care practices, and a multitude of specialty clinics. If each relevant medical establishment purchased a single CSS device, CVR Medical would be looking at a multi-billion-dollar market opportunity at $49,000 each.
The global market, then, is gargantuan—and CVR says it’s eyeing that as well, eventually.
#4 Finally, a Solid, Sober Small-Cap Dream Team in the Medical Segment
Clinical trials are huge milestones for pharmaceutical and medical device companies, and there is nothing more important than this. More fail than succeed when it comes to clinical trials, and the winners are big.
But smart investors in this segment aren’t looking for early blockbuster potential—they’re looking for solidity, efficacy and long-term market disrupting potential.
The company’s debut medical device, the Carotid Stenotic Scan (CSS), has been quietly in development for 10 years. Instead of trying to lure in investors with early stage blockbuster talk, it waited until development was real, and the long road to validation came visibly closer to the light at the end of the tunnel.
The first thing that comes to mind when you meet the team behind CVR and the development of this breakthrough technology is an unheard-of modesty and professionalism. This team has demonstrated the strategic vision of a supergiant by leveraging intellectual property, market and industry know-how and key strategic relationships.
But what is most inviting in the tricky small-cap world of pharmaceuticals and medical devices is that this team kept its CSS development quiet until recently. There was no hype and no pump. Now they are on the solid path to real validation. This alone signals a sober solidity that makes a small-cap bet on a large-cap market attractive.
Led by Chairman, CEO and President Peter Bakema, with an impressive 30-year track record in business development, since its inception, CVR (TSX:CVM.V; OTC:CRRVF) has brought on some of the most respected medical professionals in the industry.
• Tony Robinson, COO and Executive Vice-President has been with CVR for 8 years and has extensive domestic and global healthcare experience.
• Michael Rhodes, VP of Quality Systems, is a former VP for Quality for HSBC and Motorola. He has 20 years of experience in multiple markets.
• Dr. W. Douglas Weaver, a member of the BOD Scientific Advisory Board, is the former president of the American College of Cardiology and the former VP and System Medical Director of Heart and Vascular Services at Henry Ford Health System. His over 330 publications related to drug and device discovery have been some of the most influential in our time.
Together, they are on a trajectory that plans to bring a long-sought-after solution to helping detect the risk of ischemic stroke to the market in an affordable and accessible way. Their latest news also comes at a very critical time on our nation’s—and indeed, the world’s—healthcare story.
And now, following the release of updated preliminary clinical results from Thomas Jefferson University, Bakema is ready to speak out.
“You can’t come out with a lot of changes while you are validating, so we are almost over that hurdle. We are nearly moving into pivotal trials, which puts us right at the door for FDA approval,” he stated in a recent interview.
CVR now has several clinical sites where they are initiating trials at two of the leading research hospitals in the country. Doctors at both are “excited about getting CSS in there, and we have many other sites interested in conducting addional trials” says Bakema.
After a long development journey, CVR is now becoming well-known in the medical community. The potential for research is huge, and “the sky is the limit as far as the amount of news that could come out of clinical relationships we are building now,” the CVR CEO told us.
#5 Still Priced Like Early Stage Company
CVR (TSX:CVM.V; OTC:CRRVF) has already invested US$23 million into the research and development of their breakthrough CSS technology, and right now they’re at that critical juncture where the path to potential FDA approval is at least visible.
This is a small-cap, so shares are still priced as a small-cap, but once they enter pivotal trials the window of opportunity may have closed significantly. When and if the CSS clears pivotal trials and is followed by a full clinical report, FDA submission is the next step, and then—if successful—market clearance and delivery.
Because the expected all-in manufacturing and sales costs are less than half the sale proceeds, the company is expecting a very quick and lucrative head start. The team has already lined up manufacturing and components, so once the clinical reports are in, and the FDA hurdle is cleared, sales and profits could start quickly.
They’ve also got $2 million in the bank and a number of warrants being exercised.
A key indicator of where this is going came in August, when CVR announced that after years of development, it signed a letter of intent with Canon Virginia, Inc. (CVI), a domestic manufacturing subsidiary of Canon U.S.A., Inc. The deal, when fully executed, means that Cannon will manufacture the CSS at its state-of-the-art facilities. It’s a relationship that, according to Bakema, is highly important, not only for credibility, but also for scalability and reduced cost of entry to market.
Other recent manufacturing deals have also poised CVR for success right out of the gate. In March, they announced a partnership with ADCO Circuits, which will be the exclusive provider of CSS custom circuit boards for its sensors.
In combination with the recently updated preliminary clinical results, growing clinical relationships and the move towards hoped-for FDA approval, the catalysts for CVR Medical (TSX:CVM.V; OTC:CRRVF) are building—fast.
The timing couldn’t be better, for investors—or for the millions of people falling victim to stroke around the world every year.
Other pharma and biotech companies with interesting developments:
Celgene Corporation (NASDAQ:CELG): is a biopharmaceutical company based in Summit, New Jersey. Coming from humble beginnings in 1986, the company has grown tremendously through a series of high profile acquisitions, gaining skill and expanding its knowledge base along the way.
Celgene is on the cutting edge of the biopharmaceutical field. The company’s signature product, Revlimid, along with other drugs has been regarded as one of the most important treatments for myeloma, significantly improving patient’s chances of survival.
Sitting at an impressive $108B market cap, with solid leadership and a strong history of success, Celgene is definitely a company to keep your eyes on.
AbbVie Inc (NYSE:ABBV): is no stranger to the biopharmaceutical industry. Founded in 2013, after splitting off from Abbott Laboratories, the company’s forward-thinking management and promising medical developments have caught the attention of a lot of high profile investors.
AbbVie’s breakthrough technology comes from its focus on research. The company’s products include treatments for major illnesses such as human immunodeficiency virus and hepatitis C, Parkinson’s disease, multiple sclerosis, and many other. The company’s products are savings lives and making history.
With a market cap of $119B, and steady growth in 2017, AbbVie is a promising choice for investors. And it pays out dividends.
Corbus Pharmaceuticals (NASDAQ:CRBP): is a small-cap drug developer with a $400 million market cap. The company’s primary product is a synthetic oral endocannabinoid-mimetic – A drug that binds to the same receptors as cannabis. The drug mimics cannabinoid-based medications and has shown tremendous promise in reducing pain and inflammation. The drug currently being evaluated in four clinical programs: systemic sclerosis, cystic fibrosis, dermatomyositis and systemic lupus erythematosus.
As a leader in endocannabinoid-mimetic technology, Corbus is a great pick for those looking to get into bio-tech stocks at a lower price. With lots of room to grow, Corbus will be making waves in the field.
Intrexon Corp (NYSE:XON): is a leader in the bio-tech field. The company’s biologically-based products and processes are some of the most interesting and important revelations in the medical industry. Designing, building, and regulating gene programs, the company’s technological advancements are ahead of most.
One of Intrexon’s most important technologies is its patented UltraVector. The platform is a software-based operating system that accurately maps and assembles genetic programs. The platform allows for deeper understanding of how everything works and allows for experiments not possible otherwise.
As tech continues to fuel medical breakthroughs, Intrexon Corp is definitely worth keeping an eye on.
Quintiles IMS Holdings, Inc (NYSE:Q): is one of the world’s “most admired companies” according to Fortune Magazine. Spanning over 100 countries, Quintiles IMS is definitely ahead of the pack. Quintiles IMS provides research and development solutions to pharmaceutical, biotech, and medical device industries that push healthcare forward using data and technology.
Founded in 1982, it is safe to say that Quintiles IMS has been around the block a few times. The company’s strong management and forward-thinking attitude provide investors reassurance in a chaotic marketplace. As the world’s largest contract research organization, the company has key relationships throughout the global medical field, making it a strong bet for potential investors.
Aphria Inc (TSE:APH): Aphria Inc is engaged in the production and selling of medicinal marijuana, and while the stock has trended downward since April, the constant profits here suggest there is a lot of upside. The recent pro-marijuana legislation from the Canadian government is sure to boost companies with the reputation of Aphria Inc.
Aphria’s $137 million expansion project is well underway to ramp up output to 70,000 kg. If Aphria successfully ramps up production, we believe its share price could go much higher.
MedReleaf Corp (TSE:LEAF): As a licensed producer of cannabis-based pharmaceutical products, MedReleaf Corp has a head start on the coming boom in Canada. Early July has seen a bounce in the stock price, and investors may look to ride it upward from here. Med Releaf could become Canada’s second biggest medical marijuana company after Canopy Growth Cooperation as many analysts expect the ‘legal weed’ industry to grow 25 percent on an annual basis over the next 10 years.
MedReleaf has seen its share price fall since June, but has steadied out in recent months and could be poised for gains as the expected legalization of recreational marijuana materializes.
Oncolytics Biotech (TSX:ONC): Oncolytics has been testing the potential of its lead product reolysin through various stages of trials battling many types of cancer. Reolysin is the company’s cancer therapeutics that has been used alone and in combination with biologics, chemotherapy, and radiotherapy for various cancers. On that note, Oncolytics’ currently testing Reolysin treating cancers like pancreatic, breast and lung.
The company saw its share price jump significantly in May and while the share price came down in June, we see upside for this promising biotech firm.
Theratechnologies Inc (TSE:TH): The company is known best for its HIV treatment plans, aimed at increasing the quality of life and catering to the medical needs of patients suffering from HIV. The company’s main drug is Egrifta which is used in the treatment of HIV-associated lipodystrophy.
Possibly the best performing pharmaceutical stock of 2017, Theratechnologies has seen its stock soar nearly 300 percent since January 1st. The company’s incredible leadership and promising connections make it a company to believe in. It will remain a stock to watch in drug markets, and if its current growth trend continues investors will be seeing some incredible returns.
ProMetic Life Sciences Inc. (TSX:PLI)— Based in Quebec, Canada, this biopharmaceutical is on the cutting edge of medical technology. ProMetic products are used across the field in drug development, particularly in the purification of biologics.
ProMetic is the parent company of three subsidiaries covering two business segments: Protein Technologies and Small Molecule Therapeutics Technologies. With locations in Great Britain, Canada, and the United States, the company is well represented in major markets.
The $1.2 billion-market cap company has remedied its financing overhang, and also got a boost from a $9.5-million purchase order this year. While it has had a rough year to date, the price target remains positive – with several analysts believing it is currently oversold.
**IMPORTANT! BY READING OUR CONTENT YOU EXPLICITLY AGREE TO THE FOLLOWING. PLEASE
READ CAREFULLY**
DISCLAIMERS
PAID ADVERTISEMENT. This communication is a paid advertisement and is not a recommendation to
buy or sell securities. Oilprice.com, Advanced Media Solutions Ltd, and their owners, managers,
employees, and assigns (collectively “the Company”) has been paid by the profiled company or a third
party to disseminate this communication. In this case the Company has been paid by CVR Medical. This
compensation is a major conflict with our ability to be unbiased, more specifically:
This communication is for entertainment purposes only. Never invest purely based on our
communication. Gains mentioned in our newsletter and on our website may be based on end-of- day or
intraday data. If we own any shares we will list the information relevant to the stock and number of
shares here. We have been compensated by CVR Medical Corp. to conduct investor awareness
advertising and marketing for [TSX:CVM.V and OTC:CRRVF]. Oilprice.com receives financial
compensation to promote public companies. This compensation is a major conflict of interest in our
ability to be unbiased. Therefore, this communication should be viewed as a commercial advertisement
only. We have not investigated the background of the company. The third party, profiled company, or
their affiliates may liquidate shares of the profiled company at or near the time you receive this
communication, which has the potential to hurt share prices. Any non- compensated alerts are purely
for the purpose of expanding our database for the benefit of our future financially compensated
investor awareness efforts. Frequently companies profiled in our alerts experience a large increase in
volume and share price during the course of investor awareness marketing, which often end as soon as
the investor awareness marketing ceases. The investor awareness marketing may be as brief as one day,
after which a large decrease in volume and share price is likely to occur. Our emails may contain forward
looking statements, which are not guaranteed to materialize due to a variety of factors.
We do not guarantee the timeliness, accuracy, or completeness of the information on our site or in our
newsletters. The information in our communications and on our website is believed to be accurate and
correct, but has not been independently verified and is not guaranteed to be correct. The information is
collected from public sources, such as the profiled company’s website and press releases, but is not
researched or verified in any way whatsoever to ensure the publicly available information is correct.
Furthermore, The Company often employs independent contractor writers who may make errors when
researching information and preparing these communications regarding profiled companies.
Independent writers’ works are double-checked and verified before publication, but it is certainly
possible for errors or omissions to take place during editing of independent contractor writer’s
communications regarding the profiled company(s). You should assume all information in all of our
communications is incorrect until you personally verify the information, and again are encouraged to
never invest based on the information contained in our written communications.
DISCLOSURE. The Company does not make any guarantee or warranty about what is advertised above.
The Company is not affiliated with, any specific security. While the Company will not engage in front-
running or trading against its own recommendations, The Company and its managers and employees
reserve the right to hold possession in certain securities featured in its communications. Such positions
will be disclosed AND will not purchase or sell the security for at least two (2) market days after
publication.
NOT AN INVESTMENT ADVISOR. The Company is not registered or licensed by any governing body in
any jurisdiction to give investing advice or provide investment recommendation. ALWAYS DO YOUR
OWN RESEARCH and consult with a licensed investment professional before making an investment. This
communication should not be used as a basis for making any investment.
INDEMNIFICATION/RELEASE OF LIABILITY. By reading this communication, you agree to the terms of
this disclaimer, including, but not limited to: releasing The Company, its affiliates, assigns and successors
from any and all liability, damages, and injury from the information contained in this communication.
You further warrant that you are solely responsible for any financial outcome that may come from your
investment decisions.
SHARE OWNERSHIP. Share Ownership. The owner of Oilprice.com owns shares of this issuer and
therefore has an additional incentive to see the issuer’s stock perform well. The owner of Oilprice.com
will not notify the market when it decides to buy more or sell shares of this issuer in the market, but will
not trade on material information that has not been disclosed to the public. The owner of Oilprice.com
will be buying and selling shares of this issuer for its own profit. This is why we stress that you conduct
extensive due diligence as well as seek the advice of your financial advisor or a registered broker-dealer
before investing in any securities.
FORWARD-LOOKING STATEMENT. Statements in this communication which are not purely historical are
forward-looking statements and include statements regarding beliefs, plans, intent, predictions or other
statements of future tense. Forward-looking statements in this press release include that CVR’s
technology can successfully be deployed for early detection of stroke; that CVR’s technology may have a
major impact on the medical device industry; that CVR can sign a definitive manufacturing agreement to
give it state-of- the-art manufacturing capabilities with Canon Virginia, Inc and immediate scalability;
that CVR’s device offer a way to identify those at risk of a stroke and arming the healthcare provider
with the information to prevent it; that the device ca be used without the assistance of a certified
technician; that this could potentially be one of the most lucrative phenomena in recent medical
equipment market history; that the device makes testing cheap, affordable and accessible to all; that the
CSS device can be sold profitably at $49,000 per unit; that CVR is moving to pivotal trials and with that
will be closer to FDA approval; that CVR’s clinical relationships have great potential; that the
manufacturing and sales costs are expected to be less than half the sale proceeds; that CVR is not
expecting to have to raise any money until 2018; and that CVR have intellectual protection on our
technology. Actual events or results may differ materially from those projected in any of such
statements due to various factors, including the risks and uncertainties inherent in medical device
development, which include, without limitation, the potential failure of device candidates to advance
through clinical studies or demonstrate safety and efficacy in clinical testing; CVR’s ability to retain key
employees; its ability to finance development; and its ability to satisfy the rigorous regulatory
requirements for new medical devices. Good results in small trials and among limited cases does not
necessarily lead to the same good results for large numbers or in the general public. Our costs may be
higher than expected and CVR may need to increase the expected sales price of its device and/or raise
additional funds from the sale of equity prior to 2018. Competitors may develop better or cheaper
alternatives to CVR’s products. Having intellectual property rights does not guarantee that they may not
be successfully challenged, or that we may be infringing on the intellectual property of others. CVR may
not be able to come to final agreements with expected contract partners, it may not be able to
commercialize its products and even if it does, it may not realize any profit. The potential market may be
much smaller than expected. Readers are cautioned not to place undue reliance on these forward-
looking statements, which speak only as of the date hereof. All forward-looking statements are qualified
in their entirety by this cautionary statement and we undertake no obligation to revise or update this
information to reflect events or circumstances after today’s date. Readers should also refer to the risk
factors disclosure outlined in CVR’s periodic reports filed from time-to- time with the securities
regulators.
As defined in the United States Securities Act of 1933 Section 27(a), as amended in the Securities
Exchange Act of 1934 Section 21(e), statements in this communication which are not purely historical
are forward-looking statements and include statements regarding beliefs, plans, intent, predictions or
other statements of future tense.
PAST PERFORMANCE IS NOT INDICATIVE OF FUTURE RESULTS. Investing is inherently risky. While a
potential for rewards exists, by investing, you are putting yourself at risk. You must be aware of the risks
and be willing to accept them in order to invest in any type of security. Don’t trade with money you can’t
afford to lose. This is neither a solicitation nor an offer to Buy/Sell securities. No representation is being
made that any account will or is likely to achieve profits or losses similar to those discussed on this web
site. The past performance of any trading system or methodology is not necessarily indicative of future
results
CFTC RULE 4.41 – HYPOTHETICAL OR SIMULATED PERFORMANCE RESULTS HAVE CERTAIN LIMITATIONS.
UNLIKE AN ACTUAL PERFORMANCE RECORD, SIMULATED RESULTS DO NOT REPRESENT ACTUAL
TRADING. ALSO, SINCE THE TRADES HAVE NOT BEEN EXECUTED, THE RESULTS MAY HAVE UNDER-OR-
OVER COMPENSATED FOR THE IMPACT, IF ANY, OF CERTAIN MARKET FACTORS, SUCH AS LACK OF
LIQUIDITY. SIMULATED TRADING PROGRAMS IN GENERAL ARE ALSO SUBJECT TO THE FACT THAT THEY
ARE DESIGNED WITH THE BENEFIT OF HINDSIGHT. NO REPRESENTATION IS BEING MADE THAT ANY
ACCOUNT WILL OR IS LIKELY TO ACHIEVE PROFIT OR LOSSES SIMILAR TO THOSE SHOWN.
All trades, patterns, charts, systems, etc., discussed in this message and the product materials are for
illustrative purposes only and not to be construed as specific advisory recommendations. All ideas and
material presented are entirely those of the author and do not necessarily reflect those of the publisher.
No system or methodology has ever been developed that can guarantee profits or ensure freedom from
losses. No representation or implication is being made that using the methodology or system will
generate profits or ensure freedom from losses. The testimonials and examples used herein are
exceptional results, which do not apply to the average member, and are not intended to represent or
guarantee that anyone will achieve the same or similar results.
AFFILIATES. Some or all of the content provided in this communication may be provided by an affiliate of
The Company. Content provided by an affiliate may not be reviewed by the editorial staff member. Our
affiliates may have their own disclosure policies that may differ from The Company’s policy.
TERMS OF USE. By reading this communication you agree that you have reviewed and fully agree to the
Terms of Use found here http://oilprice.com/terms-and- conditions If you do not agree to the Terms of
Use http://oilprice.com/terms-and- conditions, please contact Oilprice.com to discontinue receiving
future communications.
The information contained herein may change without notice.
Source Article from http://feedproxy.google.com/~r/blacklistednews/hKxa/~3/bNbwNXZzOIM/M.html
 RSS Feed
RSS Feed















 September 12th, 2017
September 12th, 2017  Awake Goy
Awake Goy 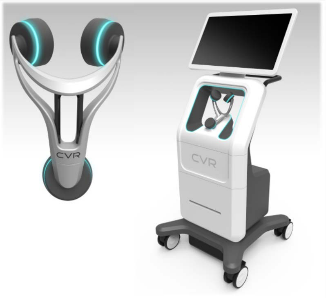
 Posted in
Posted in  Tags:
Tags: 













