According to a study released today by a board of international experts, including Marion Gruber, director of the FDA’s Office of Vaccines Research and Review, and deputy director Phil Krause, the vaccine boosters actually have a substantial risk to cause myocarditis, inflammation of the heart; and other accute reactions, as well as being totally unnecessary for the average person to have.
This spells trouble for Pfizer-Biotexh who has previously published questionable studies it funded, undercutting its own vaccine in order to push said expensive boosters to people everywhere.
Considerations in boosting COVID-19 vaccine immune responsesPhilip R Krause, MD Prof Thomas R Fleming, PhDProf Richard Peto, FRSProf Ira M Longini, PhDProf J Peter Figueroa, PhDProf Jonathan A C Sterne, PhDet al.Show all authorsPublished:September 13, 2021DOI:https://doi.org/10.1016/S0140-6736(21)02046-8PlumX MetricsDeclaration of interestsSupplementary MaterialReferencesArticle InfoFigures
 A new wave of COVID-19 cases caused by the highly transmissible delta variant is exacerbating the worldwide public health crisis, and has led to consideration of the potential need for, and optimal timing of, booster doses for vaccinated populations.1 Although the idea of further reducing the number of COVID-19 cases by enhancing immunity in vaccinated people is appealing, any decision to do so should be evidence-based and consider the benefits and risks for individuals and society. COVID-19 vaccines continue to be effective against severe disease, including that caused by the delta variant. Most of the observational studies on which this conclusion is based are, however, preliminary and difficult to interpret precisely due to potential confounding and selective reporting. Careful and public scrutiny of the evolving data will be needed to assure that decisions about boosting are informed by reliable science more than by politics. Even if boosting were eventually shown to decrease the medium-term risk of serious disease, current vaccine supplies could save more lives if used in previously unvaccinated populations than if used as boosters in vaccinated populations.
A new wave of COVID-19 cases caused by the highly transmissible delta variant is exacerbating the worldwide public health crisis, and has led to consideration of the potential need for, and optimal timing of, booster doses for vaccinated populations.1 Although the idea of further reducing the number of COVID-19 cases by enhancing immunity in vaccinated people is appealing, any decision to do so should be evidence-based and consider the benefits and risks for individuals and society. COVID-19 vaccines continue to be effective against severe disease, including that caused by the delta variant. Most of the observational studies on which this conclusion is based are, however, preliminary and difficult to interpret precisely due to potential confounding and selective reporting. Careful and public scrutiny of the evolving data will be needed to assure that decisions about boosting are informed by reliable science more than by politics. Even if boosting were eventually shown to decrease the medium-term risk of serious disease, current vaccine supplies could save more lives if used in previously unvaccinated populations than if used as boosters in vaccinated populations.
Boosting could be appropriate for some individuals in whom the primary vaccination, defined here as the original one-dose or two-dose series of each vaccine, might not have induced adequate protection—eg, recipients of vaccines with low efficacy or those who are immunocompromised2 (although people who did not respond robustly to the primary vaccination might also not respond well to a booster). It is not known whether such immunocompromised individuals would receive more benefit from an additional dose of the same vaccine or of a different vaccine that might complement the primary immune response.
Boosting might ultimately be needed in the general population because of waning immunity to the primary vaccination or because variants expressing new antigens have evolved to the point at which immune responses to the original vaccine antigens no longer protect adequately against currently circulating viruses.
Although the benefits of primary COVID-19 vaccination clearly outweigh the risks, there could be risks if boosters are widely introduced too soon, or too frequently, especially with vaccines that can have immune-mediated side-effects (such as myocarditis, which is more common after the second dose of some mRNA vaccines,3 or Guillain-Barre syndrome, which has been associated with adenovirus-vectored COVID-19 vaccines4). If unnecessary boosting causes significant adverse reactions, there could be implications for vaccine acceptance that go beyond COVID-19 vaccines. Thus, widespread boosting should be undertaken only if there is clear evidence that it is appropriate.
Findings from randomised trials have reliably shown the high initial efficacy of several vaccines, and, less reliably, observational studies have attempted to assess the effects on particular variants or the durability of vaccine efficacy, or both. The appendix identifies and describes the formal and informal reports from these studies. Some of this literature involves peer-reviewed publications; however, some does not, and it is likely that some details are importantly wrong and that there has been unduly selective emphasis on particular results. Together, however, these reports provide a partial but useful snapshot of the changing situation, and some clear findings emerge. The figure summarises the reports that estimated vaccine efficacy separately for severe disease (variously defined) and for any confirmed SARS-CoV-2 infection, plotting one against the other. A consistent finding is that vaccine efficacy is substantially greater against severe disease than against any infection; in addition, vaccination appears to be substantially protective against severe disease from all the main viral variants. Although the efficacy of most vaccines against symptomatic disease is somewhat less for the delta variant than for the alpha variant, there is still high vaccine efficacy against both symptomatic and severe disease due to the delta variant.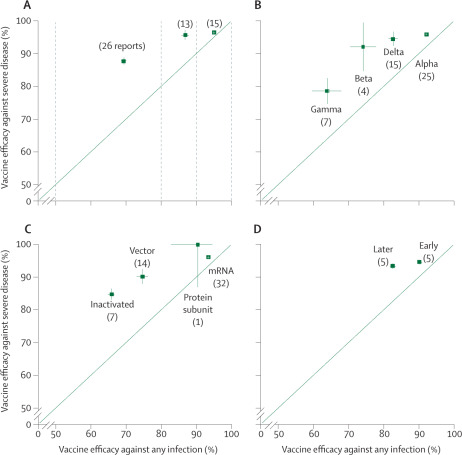 FigureVaccine efficacy against severe disease versus vaccine efficacy against any infection View Large Image Figure Viewer Download Hi-res image Download (PPT)
FigureVaccine efficacy against severe disease versus vaccine efficacy against any infection View Large Image Figure Viewer Download Hi-res image Download (PPT)
Current evidence does not, therefore, appear to show a need for boosting in the general population, in which efficacy against severe disease remains high. Even if humoral immunity appears to wane, reductions in neutralising antibody titre do not necessarily predict reductions in vaccine efficacy over time, and reductions in vaccine efficacy against mild disease do not necessarily predict reductions in the (typically higher) efficacy against severe disease. This effect could be because protection against severe disease is mediated not only by antibody responses, which might be relatively short lived for some vaccines, but also by memory responses and cell-mediated immunity, which are generally longer lived.5 The ability of vaccines that present the antigens of earlier phases of the pandemic (rather than variant-specific antigens) to elicit humoral immune responses against currently circulating variants6, 7 indicates that these variants have not yet evolved to the point at which they are likely to escape the memory immune responses induced by those vaccines. Even without any changes in vaccine efficacy, increasing success in delivering vaccines to large populations will inevitably lead to increasing numbers of breakthrough cases, especially if vaccination leads to behavioural changes in vaccinees.
Randomised trials are relatively easy to interpret reliably, but there are substantial challenges in estimating vaccine efficacy from observational studies undertaken in the context of rapid vaccine roll-out. Estimates may be confounded both by patient characteristics at the start of vaccine roll-out and by time-varying factors that are missed by electronic health records. For example, those classified as unvaccinated might include some who were in fact vaccinated, some who are already protected because of previous infection, or some whose vaccination was deferred because of COVID-19 symptoms. The likelihood that there are systematic differences between vaccinated and unvaccinated individuals may increase as more people get vaccinated and as patterns of social interaction between vaccinated and unvaccinated people change. Apparently reduced efficacy among people immunised at the beginning of the pandemic could also arise because individuals at high risk of exposure (or of complications) were prioritised for early immunisation. Among vaccinated people, more of the severe disease could be in immunocompromised individuals, who are plausibly more likely to be offered and seek vaccination even though its efficacy is lower than it is in other people.2 Test-negative designs, which compare vaccination status of people who tested positive and those who tested negative, can sometimes reduce confounding,8 but do not prevent distortion of results due to the so-called collider bias.9 The probability that individuals with asymptomatic or mild COVID-19 infection will seek testing might be influenced by whether they are vaccinated. In addition, outcomes may be affected over time by varying stress on health-care facilities. However, careful observational studies that examine efficacy against severe disease remain useful and are less likely to be affected by diagnosis-dependent biases over time than are observational studies of milder disease, and could therefore provide useful indicators of any changes in vaccine-induced protection.
To date, none of these studies has provided credible evidence of substantially declining protection against severe disease, even when there appear to be declines over time in vaccine efficacy against symptomatic disease. In a study in Minnesota, USA,10 point estimates of the efficacy of mRNA vaccines against hospitalisation appeared lower in July, 2021, than in the previous 6 months, but these estimates had wide confidence intervals and could have been affected by some of the issues described above. Of interest, reported effectiveness against severe disease in Israel was lower among people vaccinated either in January or April than in those vaccinated in February or March,11 exemplifying the difficulty of interpreting such data. A recent report on the experience in Israel during the first 3 weeks of August, 2021, just after booster doses were approved and began to be deployed widely, has suggested efficacy of a third dose (relative to two doses). Mean follow-up was, however, only about 7 person-days (less than expected based on the apparent study design); perhaps more importantly, a very short-term protective effect would not necessarily imply worthwhile long-term benefit.12 In the USA, large numbers of adults are fully vaccinated, large numbers are unvaccinated, and systematic comparisons between them are ongoing. Recent reports of large US studies (one from the US CDC’s COVID-NET13 and two from major health maintenance organisations14, 15) demonstrate the continued high efficacy of full vaccination against severe disease or hospitalisation.
Although vaccines are less effective against asymptomatic disease or against transmission than against severe disease, even in populations with fairly high vaccination rates the unvaccinated are still the major drivers of transmission and are themselves at the highest risk of serious disease.16 If new variants that can escape the current vaccines are going to evolve, they are most likely to do so from strains that had already become widely prevalent. The effectiveness of boosting against the main variants now circulating and against even newer variants could be greater and longer lived if the booster vaccine antigen is devised to match the main circulating variants.6 There is an opportunity now to study variant-based boosters before there is widespread need for them. A similar strategy is used for influenza vaccines, for which each annual vaccine is based on the most current data about circulating strains, increasing the likelihood that the vaccine will remain effective even if there is further strain evolution.
The message that boosting might soon be needed, if not justified by robust data and analysis, could adversely affect confidence in vaccines and undermine messaging about the value of primary vaccination. Public health authorities should also carefully consider the consequences for primary vaccination campaigns of endorsing boosters only for selected vaccines. Booster programmes that affect some but not all vaccinees may be difficult to implement—so it will be important to base recommendations on complete data about all vaccines available in a country, to consider the logistics of vaccination, and to develop clear public health messaging before boosting is widely recommended.
If boosters (whether expressing original or variant antigens) are ultimately to be used, there will be a need to identify specific circumstances in which the direct and indirect benefits of doing so are, on balance, clearly beneficial. Additional research could help to define such circumstances. Furthermore, given the robust booster responses reported for some vaccines, adequate booster responses might be achievable at lower doses, potentially with reduced safety concerns. Given the data gaps, any wide deployment of boosters should be accompanied by a plan to gather reliable data about how well they are working and how safe they are. Their effectiveness and safety could, in some populations, be assessed most reliably during deployment via extremely large-scale randomisation,17 preferably of individuals rather than of groups.
Thus, any decisions about the need for boosting or timing of boosting should be based on careful analyses of adequately controlled clinical or epidemiological data, or both, indicating a persistent and meaningful reduction in severe disease, with a benefit–risk evaluation that considers the number of severe cases that boosting would be expected to prevent, along with evidence about whether a specific boosting regimen is likely to be safe and effective against currently circulating variants. As more information becomes available, it may first provide evidence that boosting is needed in some subpopulations. However, these high-stakes decisions should be based on peer-reviewed and publicly available data and robust international scientific discussion.
The vaccines that are currently available are safe, effective, and save lives. The limited supply of these vaccines will save the most lives if made available to people who are at appreciable risk of serious disease and have not yet received any vaccine. Even if some gain can ultimately be obtained from boosting, it will not outweigh the benefits of providing initial protection to the unvaccinated. If vaccines are deployed where they would do the most good, they could hasten the end of the pandemic by inhibiting further evolution of variants. Indeed, WHO has called for a moratorium on boosting until the benefits of primary vaccination have been made available to more people around the world.18 This is a compelling issue, particularly as the currently available evidence does not show the need for widespread use of booster vaccination in populations that have received an effective primary vaccination regimen.
Contributors
All authors agree with the viewpoints expressed in the article, and contributed to conceptualisation, analysis, review, and editing. PRK wrote the original draft. IB, JPTH, FK, PRK, and HP verified the data.
Declaration of interests
We declare no competing interests.
Acknowledgments
JPTH is a senior investigator (NF-SI-0617-10145) supported by the National Institute for Health Research (NIHR) Bristol Biomedical Research Centre and NIHR Applied Research Collaboration West. JACS is supported by the NIHR Bristol Biomedical Research Centre and grant funding from the NIHR and UK Research and Innovation. IML is supported by the National Institute of Allergy and Infectious Diseases (NIAID) grants R01 AI 139761 and R56 AI 148284. TRF is supported by NIAID R37 AI 29168. The COVID-NMA initiative is supported by the Agence Nationale de la Recherche (ANR), WHO, and the French Ministry of Health. The opinions expressed are those of the authors, and do not necessarily represent the opinions of their respective organisations. The authors thank the following members of the COVID-NMA initiative (http://www.covid-nma.com) for their support with the identification of eligible studies and the extraction of the data: Carolina Graña (Université de Paris, Cochrane France), Hillary Bonnet (Université de Paris, Cochrane France), Mauricia Davidson (Université de Paris, Cochrane France), Gemma Villanueva (Cochrane response), Hanna Bergman (Cochrane response), Brian Buckley (Cochrane response), Elise Cogo (Cochrane response), Katrin Probyn (Cochrane response), Yanina Sguassero (Cochrane response), Jennifer Petkovic (Cochrane response).Supplementary Material Download .pdf (.66 MB)
 RSS Feed
RSS Feed













 September 16th, 2021
September 16th, 2021  Awake Goy
Awake Goy  Posted in
Posted in  Tags:
Tags: 
















