Memic Innovative Surgery, a medical device company, said it has received marketing authorization from the US Food and Drug Administration for a robotic surgical device it has developed to perform transvaginal hysterectomies in patients.
The Hominis Surgical System developed by Memic features miniature humanoid-shaped robotic arms. It is intended for benign hysterectomy, or the removal of the uterus for non-cancerous conditions along with the removal of one or both fallopian tubes and ovaries.
“We are providing physicians and patients another minimally-invasive gynecologic surgical option for non-cancerous conditions,” said Dr. Binita Ashar, director of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, in a statement on March 1. “The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures.”
Robotically assisted (RASD) surgical devices are not actually robots, the FDA explained in the statement. The devices cannot perform surgery without direct human control. RASD enable a surgeon to use computer and software technology to control and move surgical instruments through one or more tiny incisions in the patient’s body in a variety of surgical procedures or operations.
To remove the uterus, the Hominis Surgical System uses minimally invasive surgical instruments inserted through the vagina (transvaginal approach) and a video camera inserted laparoscopically through a small incision on the abdomen for visualization of the instruments inside the patient. The transvaginal approach requires fewer incisions on the abdomen compared to conventional laparoscopic hysterectomy. During the procedure, surgeons in the operating room control the instruments from the Hominis Surgical System console.
The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device, the statement said.
In addition to its assessment of performance and engineering testing, the FDA evaluated safety and effectiveness in a clinical study of 30 patients undergoing transvaginal total hysterectomy with the removal of one or both fallopian tubes and ovaries, for benign conditions, using the Hominis Surgical System, the statement said.
All 30 procedures “were successfully completed,” the FDA said. Adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina that is done as part of a hysterectomy.
Approximately 600,000 hysterectomies are performed annually in the US. The transvaginal approach – as opposed to abdominal hysterectomies – is documented as resulting in better clinical benefits, including less patient pain and scarring, shorter recovery times and reduced infection rates, compared to other approaches to hysterectomy. But surgeons use the transvaginal approach in only 16 percent of hysterectomies due to anatomical barriers and accessibility challenges, Memic said in its own statement.
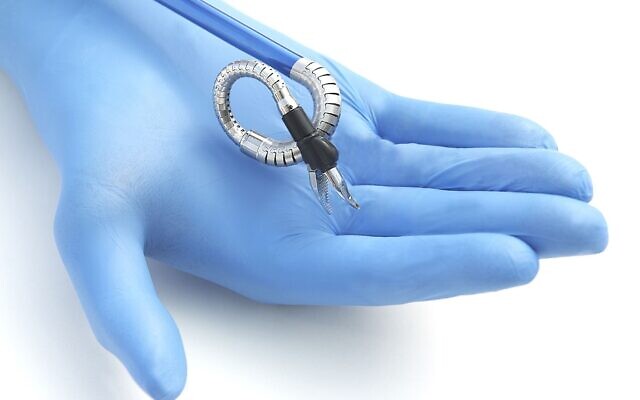
The Hominis Surgical System developed by Memic Innovative Surgery is a robotic surgical device (Courtesy)
The FDA authorization is based on clinical data from procedures that used the Hominis system on patients in Belgium and Israel.
“This FDA authorization represents a significant advance in the world of robot-assisted surgery and fulfills an unmet need in the world of robotic gynecological surgery,” said Prof. Jan Baekelandt, a gynecologist at Imelda Hospital in Bonheiden, Belgium, who performed the first hysterectomy using the Hominis system in November 2018. “Research shows vaginal hysterectomy provides optimal clinical benefits to patients including reduced pain, recovery time and rates of infection but in most countries the incidence of vaginal hysterectomy is decreasing. In addition, transvaginal approaches result in no visible scars, which is very important to the gynecological patient. Hominis is the only robot specifically developed for transvaginal surgery and is therefore small and flexible enough to perform surgery through a small incision.”
The Hominis (Latin for humanoid) system features miniature humanoid-shaped robotic arms that provide human-level dexterity, multi-planar flexibility and 360 degrees of movement, the statement said. The biomimetic instruments are designed to replicate the motions and capabilities of a surgeon’s arms, with shoulder, elbow and wrist joints. Multiple instruments can be introduced to the body through a single portal and the 360-degree articulation offers obstacle avoidance as well as optimal access and working angles.
The system will be be sold “at a significantly lower price compared to other marketed robotic surgery systems, potentially allowing more medical facilities including hospitals and ambulatory surgical centers to access and adopt surgical robotics,” the statement said.

The Hominis Surgical System developed by Memic Innovative Surgery is a robotic surgical device (Courtesy)
The company plans to expand the use of the Hominis platform for other general surgery uses as well, and is developing artificial intelligence-enabled features to support all its surgical indications.
The Hominis system “offers a small, cost-effective and less invasive option over current robotic instruments limited to straight shaft and single wrist designs and controlled with large, complex and expensive equipment,” said Dvir Cohen, CEO of Memic. “This authorization is also just the beginning; it opens the door for our novel system to expand to additional indications that, until now, have been off-limits to robot-assisted surgery.”
“We believe the Hominis surgical system represents the most significant advancement in robotic soft tissue surgery in the last 20 years and will deliver great benefit to patients and their healthcare providers,” added Maurice R. Ferré, MD, chairman of the board at Memic and founder of MAKO Surgical, Inc.
Founded in 2012 by Nir Shvalb, the firm has headquarters in Tel Aviv as well as a wholly owned subsidiary in Fort Lauderdale, Florida. The firm has received seed funding of some $950,000 from investors including Accelmed, OurCrowd and Peregrine Ventures, according to Start-Up Nation Central.
 RSS Feed
RSS Feed















 March 3rd, 2021
March 3rd, 2021  Awake Goy
Awake Goy  Posted in
Posted in  Tags:
Tags: 













